J Infect Dis:药物增强剂COBI可提高齐多夫定的抗HIV疗效
2013-04-11 J Infect Dis dxy
两组病人中HIIV-1 RNA<50 copies/mL的百分比 低剂量的药物增强剂利托那韦(RTV)能够增强蛋白酶抑制剂(PI)的活性,从而在HIV感染的治疗中起到非常重要的作用。通过增加药物动力学作用,药物增强剂可使其他药物持久有效,不易耐药,减少剂量和用药频率。Cobicistat(COBI)作为一种新的药物增强剂,已经通过2期试验,为了比较COBI和RTV在早期HIV病人治疗中对A
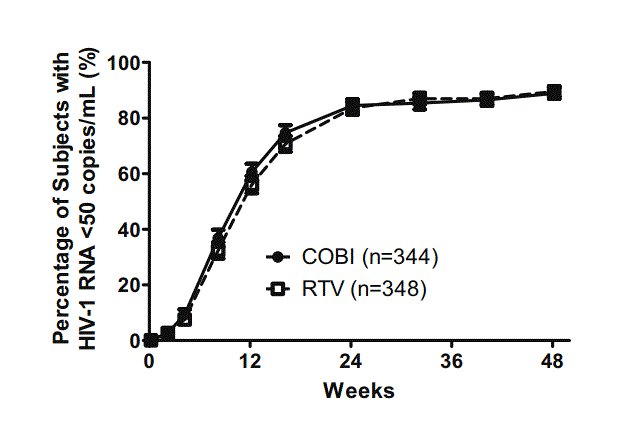
两组病人中HIIV-1 RNA<50 copies/mL的百分比
低剂量的药物增强剂利托那韦(RTV)能够增强蛋白酶抑制剂(PI)的活性,从而在HIV感染的治疗中起到非常重要的作用。通过增加药物动力学作用,药物增强剂可使其他药物持久有效,不易耐药,减少剂量和用药频率。Cobicistat(COBI)作为一种新的药物增强剂,已经通过2期试验,为了比较COBI和RTV在早期HIV病人治疗中对ATV(齐多夫定)联合FTC(恩曲他滨)/TDF(替诺福韦酯)的增强效果,来自于美国霍普金斯大学医学院的Joel E. Gallant等进行了一项研究,研究结果发表在2013年3月26日的《Journal of Infectious Diseases》杂志上。研究发现,COBI在抗HIV治疗中是一种有效的药物增强剂。
该研究是一项随机、双盲、双模拟试验。研究者选取了未经抗逆转录病毒药物治疗的HIV-1感染者,并随机将病人分成2组,在接受ATV、FTC/TDF治疗时,分别加入COBI和RTV。将48周窗口期(309-378天)内病人HIV RNA<50copies/mL的定义为病毒学成功,而将HIV RNA≥50copies/mL和无数据的定义为病毒学失败。
研究结果如下:在692名(344 COBI vs 348RTV)病人中,COBI组和RTV组中分别有85%和87%的患者达到病毒学成功(差异:-2.2%;95 CI:-7.4 to 3.0),而对于基准HIV RNA>100000 copies/mL的病人来说,两组的病毒学成功率相当(86% vs 86%)。另外,两种治疗方案产生严重副反应的比例(10%vs7% of RTV))和严重副反应导致停药的百分比(7% vs 7%)相似,血肌酐含量分别升高了0.13和0.009 mg/dL。
研究发现,在48周窗口期内,对于使用ATV联合FTC/TDF治疗的HIV-1患者中,COBI 和RTV 的药物增强效果相当。两种方案都能达到高比例的病毒学成功,而且两种治疗方案的安全性和耐受性也相似。因此,对于蛋白酶抑制剂ATV来说,每日一次的COBI是一种安全有效的药物增强剂。
与HIV相关的拓展阅读:
- J Vir:我国艾滋病疫苗能有效控制HIV病毒通过粘膜途径感染
- JAMA Intern Med:HIV感染者易发心梗
- Science:研究显示进行针对HIV的抗逆转录病毒疗法是值得的
- Neurology:HIV感染者 早诊早治有助减少神经认知损害
- Neurology:HIV感染者 早诊早治有助减少神经认知损害
- JNCI:HIV阳性者非黑色素瘤皮肤癌发病率高 更多信息请点击:有关HIV更多资讯

Cobicistat versus ritonavir as a pharmacoenhancer for atazanavir plus emtricitabine/tenofovir DF in treatment-naive HIV-1-infected patients: Week 48 results.
Background
Cobicistat (COBI) is a pharmacoenhancer with no antiretroviral activity in vitro.
Methods
An international, randomized, double-blind, double-dummy, active-controlled trial was conducted to evaluate the efficacy and safety of COBI versus ritonavir (RTV) as a pharmacoenhancer of atazanavir (ATV) in combination with emtricitabine (FTC)/ tenofovir disoproxil fumarate (TDF) in treatment-naïve patients. Primary endpoint was HIV-1 RNA<50 copies/mL at Week 48 by FDA snapshot algorithm; non-inferiority margin was 12%.
Results
A total of 692 patients were randomized and received study drug (344 COBI vs 348 RTV group). At Week 48, virologic success was achieved in 85% (COBI) and 87% (RTV) (difference: −2.2%; 95% CI: −7.4 to 3.0); among patients with baseline HIV-1 RNA >100,000 copies/mL, rates were similar (86% vs 86%). Similar percentages of patients in both groups had serious adverse events (10% vs 7%) and adverse events leading to study drug discontinuation (7% vs 7%). Median increases in serum creatinine were 0.13 and 0.09 mg/dL, respectively.
Conclusions
COBI was non-inferior to RTV in combination with ATV plus FTC/TDF at Week 48. Both regimens achieved high rates of virologic success. Safety and tolerability profiles of the two regimens were comparable. Once-daily COBI is a safe and effective pharmacoenhancer of the protease inhibitor ATV.
作者:J Infect Dis
版权声明:
本网站所有注明“来源:梅斯医学”或“来源:MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明“来源:梅斯医学”。其它来源的文章系转载文章,本网所有转载文章系出于传递更多信息之目的,转载内容不代表本站立场。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言





#Dis#
42