ACS Nano:新型诊断成像技术助化学疗法杀灭前列腺癌细胞
2012-08-14 T.Shen 生物谷
通过对人类和小鼠前列腺癌细胞进行研究,来自约翰霍普金斯大学的癌症成像专家开发出了一种新型的方法来用于发现和消灭恶性的肿瘤细胞。这种方法名为治疗诊断学成像,其可以直接打靶和追踪潜在的药物疗法来专门消灭癌细胞。这种新方法依赖于结合在化疗药物的钝化形式上,来最终吸附于肿瘤细胞表面的特殊蛋白上,最终检测药物对肿瘤的作用,这种高度的药物-蛋白复合物(或者nanoplex)的结合允许其进入癌细胞的内部来发挥杀

通过对人类和小鼠前列腺癌细胞进行研究,来自约翰霍普金斯大学的癌症成像专家开发出了一种新型的方法来用于发现和消灭恶性的肿瘤细胞。这种方法名为治疗诊断学成像,其可以直接打靶和追踪潜在的药物疗法来专门消灭癌细胞。这种新方法依赖于结合在化疗药物的钝化形式上,来最终吸附于肿瘤细胞表面的特殊蛋白上,最终检测药物对肿瘤的作用,这种高度的药物-蛋白复合物(或者nanoplex)的结合允许其进入癌细胞的内部来发挥杀伤效应。
相关研究成果刊登在了近日的国际杂志ACS Nano上,这是首次揭示了化疗法可以在分子水平被精确控制来最大化的杀死肿瘤细胞,同时尽可能减小副作用。在治疗诊断学成像实验中,研究者仅仅研究了针对癌细胞的药物,尤其是针对那些前列腺特异性膜抗原(PSMA)或者其细胞表面蛋白。
研究者Bhujwalla表示,我们的结果展示了非侵入性的成像方法以及针对表达PSMA的癌细胞的靶位疗法。新技术可以针对任何形式的癌症,包括携带有HER-2/4的乳腺癌、某些肝癌、肺癌以及可以表达特殊蛋白质的肾脏癌等。PSMA是在大部分的实体瘤血管中所表达的,意味着新型疗法应当进行一般的成像和疗法来针对癌症。
在最近的一项实验中,通过给小鼠注射前列腺癌肿瘤细胞,研究者通过成像技术来追踪抗癌药物作用于肿瘤的过程。不同的新型药物以放射性或者荧光分子为标签,混合于癌症细胞中,有些有额外的PSMA。后续试验发现注射入三种不同浓度的药物均不会给小鼠器官带来损伤效应。
研究者Pomper最后表示,我们的治疗诊断学成像方法揭示了最好的检测和治疗方法结合特异性的化学疗法所表现出来的癌症治疗的最佳效果。运用新方法,我们可以攻击多重的癌细胞,提高药物的效用。
编译自:'Theranostic' Imaging Offers Means of Killing Prostate Cancer Cells

doi:10.1021/nn301725w
PMC:
PMID:
PSMA-Targeted Theranostic Nanoplex for Prostate Cancer Therapy
Zhihang Chen †, Marie-France Penet †, Sridhar Nimmagadda †‡, Cong Li †, Sangeeta R. Banerjee †, Paul T. Winnard , Jr.†, Dmitri Artemov †‡, Kristine Glunde †‡, Martin G. Pomper †‡, and Zaver M. Bhujwalla †‡*
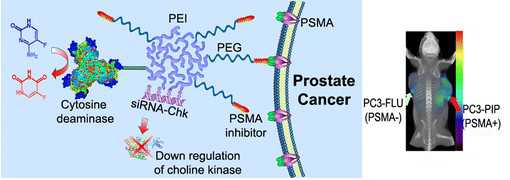
Theranostic imaging, where diagnosis is combined with therapy, is particularly suitable for a disease that is as complex as cancer, especially now that genomic and proteomic profiling can provide an extensive “fingerprint” of each tumor. With such information, theranostic agents can be designed to personalize treatment and minimize damage to normal tissue. Here we have developed a nanoplex platform for theranostic imaging of prostate cancer (PCa). In these proof-of-principle studies, a therapeutic nanoplex containing multimodal imaging reporters was targeted to prostate-specific membrane antigen (PSMA), which is expressed on the cell surface of castrate-resistant PCa. The nanoplex was designed to deliver small interfering RNA (siRNA) along with a prodrug enzyme to PSMA-expressing tumors. Each component of the nanoplex was carefully selected to evaluate its diagnostic aspect of PSMA imaging and its therapeutic aspects of siRNA-mediated down-regulation of a target gene and the conversion of a prodrug to cytotoxic drug, using noninvasive multimodality imaging. Studies performed using two variants of human PC3-PCa cells and tumors, one with high PSMA expression level and another with negligible expression levels, demonstrated PSMA-specific uptake. In addition, down-regulation of the selected siRNA target, choline kinase (Chk), and the conversion of the nontoxic prodrug 5-fluorocytosine (5-FC) to cytotoxic 5-fluorouracil (5-FU) were also demonstrated with noninvasive imaging. The nanoplex was well-tolerated and did not induce liver or kidney toxicity or a significant immune response. The nanoplex platform described can be easily modified and applied to different cancers, receptors, and pathways to achieve theranostic imaging, as a single agent or in combination with other treatment modalities.
作者:T.Shen
版权声明:
本网站所有注明“来源:梅斯医学”或“来源:MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明“来源:梅斯医学”。其它来源的文章系转载文章,本网所有转载文章系出于传递更多信息之目的,转载内容不代表本站立场。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言




#化学疗法#
61
#癌细胞#
0
#ACS#
59
#成像技术#
61
#前列腺癌细胞#
70